As the second year of the COVID-19 pandemic draws to a close, resilience—the ways in which health, economic, and environmental systems face changes and shocks by adapting, evolving, and innovating—has emerged as a crucial attribute of societies working toward a “new normal” of post-pandemic life.
Throughout 2021, the development and rollout of vaccines against COVID-19 have been crucial for reducing the impact of successive outbreaks throughout the world. However, many regions have encountered challenges in producing, distributing, and administering these life-saving vaccines.
Developed for a broad global audience, this report focuses on vaccines as key to resilience in the Asia Pacific. In what is still a fast-moving environment, this report draws on interviews with regional experts and additional research in late 2021 to identify factors in the Asia Pacific that have affected vaccine equity and rollout during the pandemic and actions that will contribute to resilience in the future. Key findings are as follows:
The “Triple Challenge”
Countries in the Asia-Pacific region currently face a “triple challenge” of (1) pursuing equitable distribution of vaccines, (2) ensuring that rollout programs successfully deliver shots into arms, and (3) developing agile manufacturing capacity to produce essential drugs and vaccines. If not addressed, these ongoing challenges may undermine regional resilience. All three dimensions of this triple challenge have contributed to the Asia Pacific’s slow progress in vaccination compared with Europe and the Americas.
The Region is Taking Action
Our contributors noted that COVID-19 is now becoming endemic, meaning that the virus will continue to adapt, circulate, and cause outbreaks into the future. This means that countries in the Asia Pacific must adapt as COVID-19 continues to affect individuals, communities, businesses, and governments across the region. The research for this report revealed that countries in the Asia Pacific have acted in 2021 to address each dimension of the triple challenge, focused on both short- and long-term resilience.
This report shows that the concept of resilience can be used as a framework to better understand the ways in which health systems in the Asia Pacific have adapted, evolved, and innovated over the course of the pandemic. Although this report has been written in a fast-moving environment—where almost two years into the pandemic, cases, hospitalizations, and deaths still vary daily from country to country—lessons have emerged about the value of regional approaches to address the triple challenge.
“We need a regional approach to complement a global approach.”
Vaccines are a key to entering the post-pandemic world. However, the ultimate solution for preventing another pandemic and the crises that ensue lies in governments, businesses, academics, and civil society committing to building health systems that can adapt, evolve, and innovate quickly and coherently in the face of these new challenges.
Therefore, as COVID-19 becomes endemic, this report proposes three foundations for resilience:
This report is made possible by support from the Fubon Cultural and Educational Foundation.
The full report is published and discussed on October 26, 2021 during our online event Vaccination and beyond: Lessons for ending the pandemic from the Asia-Pacific, Europe, and the U.S.
The Reform for Resilience Commission was created in 2020, at the onset of the COVID-19 pandemic, to foster collaboration among global leaders in the public and private sectors in order to strengthen the world’s resilience in public health, economic recovery, and environmental sustainability.
In June 2021, the Commission published its first research report on the challenges related to the pandemic. The report was produced in collaboration with the Commission’s research partners, commissioners, and hubs in the Asia Pacific, the United States, and Europe. The co-chairs and the global convener also released a statement calling for cooperative global efforts to fight the pandemic.
Now half a year later, the global crisis created by the COVID-19 pandemic is not subsiding, and low vaccination coverage continues to be the most important issue facing the world as it struggles to halt the spread of the virus and its variants. Several Asia-Pacific countries demonstrated excellent management early in the pandemic, but the region has now fallen behind in vaccinating enough of its population. As we now have learned, no one is safe until everyone is safe. Moreover, the Asia Pacific’s crucial role in the global economy means that delayed recovery here will delay recovery elsewhere.
Focusing on future resilience is now crucial as COVID-19 becomes endemic—continuing to circulate in the global population. For this reason, the Commission’s Asia-Pacific Hub has released this report ahead of critical meetings of the G20, COP26, and the World Health Assembly in the coming months to highlight the challenges facing the Asia Pacific in acquiring and delivering vaccines and identify what is needed for immediate and longer-term solutions to enhance resilience in the region. We believe this report on containing the COVID-19 pandemic in the region through enhanced vaccination efforts is the beginning of a global transformation to a more resilient world.
We would like to acknowledge the contributions made by all those involved in the founding of the Reform for Resilience Commission and its Asia-Pacific Hub: the Commission’s convenor, George Freeman, MP, its co-chairs, the commissioners, and the members of the Hub’s steering committee and advisory board. Without the enthusiastic encouragement and engagement of Irene Chen, Daniel Tsai, and David Tawei Lee, the Hub would never have been established and this research would never have been possible.
We appreciate comments provided by Dr. Danielle Cannon of Cambridge Public Health, Stefan Weber of AstraZeneca, Professor Diane Stone and Dr. Jonas Brendebach of the European University Institute, and Kim Beng Phar. Finally, a very special thank you to Alistair Lang of the Commission’s London Secretariat, as well as to John Li and Cecilia Yin from the Hub’s office in Taipei for their support.
The work of the Asia-Pacific Hub has been made possible by generous funding from the Fubon Cultural and Educational Foundation.

Co-Chair, Reform for Resilience Commission

Commissioner, Reform for Resilience Commission and Chair, Asia-Pacific Hub

Chair, International Advisory Board, Asia-Pacific Hub

Co-chair, Resilience Commission
Chair, Gavi, the Vaccine Alliance

Commissioner and Chair of the Asia-Pacific Hub, Resilience Commission
Compton Visiting Professor, Miller Center, University of Virginia

Co-chair, Cambridge Public Health
University of Cambridge

Commissioner, Resilience Commission
Director General, Confindustria, 2012–2020

Chair, International Advisory Board, Asia-Pacific Hub
Chair Professor of Public Health, National Taiwan University

Professor of Law, Public Health Sciences, and Public Policy, University of Virginia

Commissioner, Resilience Commission
Distinguished Professor, Genomics Research Center, Academia Sinica

Commissioner, Resilience Commission
Co-founder and Honorary Chairman, Acer Inc.

Executive Director, North American Hub, Resilience Commission
Research Scientist, Harvard T.H. Chan School of Public Health

Advisor, Asia-Pacific Hub
Dean, National University of Singapore Saw Swee Hock School of Public Health

Advisor, Asia-Pacific Hub, Resilience Commission
University Professor and Professor of Public Policy, University of Virginia

Co-chair, Resilience Commission
Prime Minister of Australia, 2015–2018

Professor, School of Transnational Governance, European University Institute

Co-chair, Resilience Commission
Dean, Harvard T.H. Chan School of Public Health

Commissioner, Resilience Commission
Professor of Public Health and Director, Centre for Health Systems and Policy Research, The Chinese University of Hong Kong
Dr. Anneke Schmider
Research Director, Asia-Pacific Hub, Reform for Resilience Commission
Siwei Huang
Research Associate, Asia-Pacific Hub, Reform for Resilience Commission
Caroline Fried
Research Associate, Asia-Pacific Hub, Reform for Resilience Commission
Vaccines have emerged as key to resilience as the second year of the COVID-19 pandemic draws to a close, underpinning the ways in which health, economic, and environmental systems face changes and shocks by adapting, evolving, and innovating.
Equity in vaccine access and rollout is crucial for societies working toward a “new normal” of post-pandemic life. Global access to vaccines is widely understood to be inequitable, and the COVID-19 pandemic has revealed a need to determine how vital medical supplies, drugs, and vaccines should be shared equitably in an emergency.
As the global supply of vaccines has gradually increased to meet demand throughout 2021, the question of what constitutes vaccine equity—both among and within countries—has not been answered. Justifiable arguments for different prioritizing strategies have been made, and vaccination programs began in the midst of this debate.[1] No one yet has an answer to how need should be prioritized, and all parts of the globe, including the Asia Pacific, are still debating this as societies push for vaccination efforts. Given these unresolved ethical discussions, this report provides an overview of vaccination progress in the Asia Pacific to date and challenges facing the region.
Countries in the Asia Pacific welcomed the rollout of vaccines as a crucial part of ending the COVID-19 pandemic and enhancing resilience. However, vaccination progress in the Asia Pacific is highly varied, and in several jurisdictions, lags other regions such as Europe and the Americas (Figure 1).[2] The shares of people fully vaccinated in the region range from almost 80% in Singapore to less than 2% in Papua New Guinea at the time of writing. Even though Asia and the Pacific (Oceania) had fully vaccinated around 40% of their populations as of October 17, 2021 (compared with more than 50% in North America and over 60% in the European Union), these averages mask significant country-level variation in vaccination coverage.
Figure 1. Shares of people fully vaccinated against COVID-19 as of October 17, 2021.
Data on population vaccination coverage can be used only to compare progress in vaccination rollout between countries. These data do not indicate whether countries have reached appropriate coverage to protect their populations or to resume “normal life.”
“More research is now needed to define variables such as ‘herd immunity’ and to develop risk assessment frameworks for policy.”
As our contributors noted, no consensus has yet been reached on targets for vaccination coverage for a population, despite the fact that such targets are crucial for policymaking and future resilience. Given the spread of the Delta variant, current vaccination policy targets in some countries in the Asia Pacific are increasing to around 80%. However, with immunity against COVID-19 still the subject of substantial study, even with high vaccine coverage, other public health measures and better surveillance mechanisms must continue to remain in place. This makes vaccines the foundation, but not the entire answer, to exiting the pandemic.
Within the Asia-Pacific region, our research shows that intercountry differences in vaccination progress do not always reflect socioeconomic differences among those countries. For example, Figure 2 (below) presents the share of people fully vaccinated against COVID-19 in high-income economies in the Asia Pacific, with data showing that even some wealthy economies are struggling to vaccinate their populations.
Within the Asia-Pacific region, our research shows that intercountry differences in vaccination progress do not always reflect socioeconomic differences among those countries. For example, Figure 2 (below) presents the share of people fully vaccinated against COVID-19 in high-income economies in the Asia Pacific, with data showing that even some wealthy economies are struggling to vaccinate their populations.
Singapore, a high-income economy with a small population, fully vaccinated approximately 80% of its population by October 2021.
However, other high-income economies, including Australia, Japan, and New Zealand, have been unable to rapidly vaccinate their populations, even though they have successfully controlled the spread of COVID-19 and have excellent public health systems. These countries are only catching up to Singapore.
In Taiwan, where public vaccination efforts finally began in mid-2021, vaccination coverage is still below the Asian average.
Figure 2. Share of people fully vaccinated against COVID-19 in high-income Asia-Pacific economies as of October 17, 2021.
Further analysis of low- and middle-income countries in the region also reveals wide variation in vaccination coverage (Figure 3). For example,
China, Malaysia, Maldives, and Fiji (upper middle–income economies) and Cambodia and Sri Lanka (lower middle–income economies) have the largest portions of their populations fully vaccinated. Cambodia’s success in vaccination has been attributed to its small population, early procurement, and swift rollout program. Cambodia’s experience is revisited later in this report.
By contrast, Cambodia’s neighbors Vietnam and Laos, as well as many other Asian middle-income economies, have lagged in vaccination, currently with full vaccination percentages below the regional average.
Figure 3. Share of people fully vaccinated against COVID-19 in middle-income Asia-Pacific economies as of October 17, 2021.
According to the Economist Intelligence Unit (EIU), the majority of countries in the Asia Pacific are facing delayed vaccination timelines compared with advanced economies in North America and Europe, which will vaccinate the majority (60%–70%) of their populations by late 2021 (Figure 4).[3]
In the Asia-Pacific region, Australia, Malaysia, and New Zealand are likely to reach this vaccination benchmark by mid-2022. However, many other countries in the region, including India and Thailand, will not be able to vaccinate majority of their populations until late 2022.
For populous countries like Indonesia and the Philippines, as well as middle- and lower-income countries like Bangladesh, Laos, Myanmar, and Papua New Guinea, even the 60% vaccination target remains unattainable even by 2023, putting them at the same disadvantage African countries face in their vaccination efforts.
These trends in vaccination coverage will affect when individual countries—and therefore, the world—will exit the pandemic. Delayed vaccination will cost the region substantially; the EIU estimated that 73% of global GDP losses in 2022–2025 due to delayed vaccinations will be incurred in the Asia Pacific. The region’s crucial role in global supply chains, manufacturing, and commerce means that the Asia Pacific’s recovery from the pandemic will be instrumental to the recovery of the world economy.
Figure 4. Forecasted pace of vaccination as of July 28, 2021.
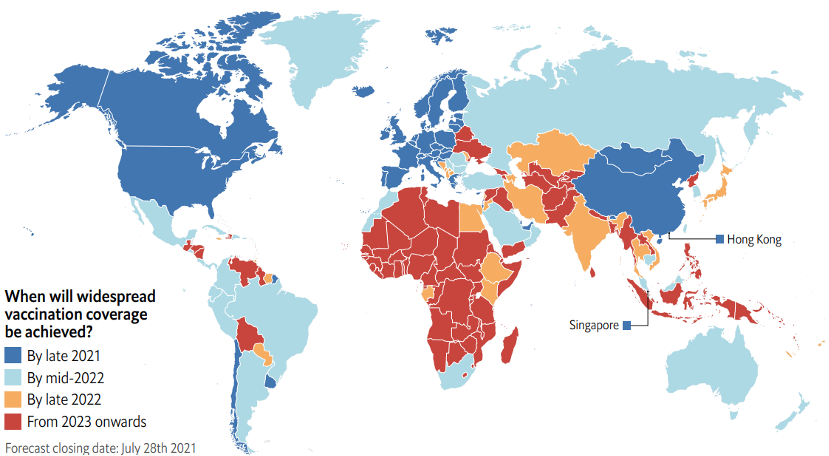
Source: The Economist Intelligence Unit
COVID-19 is now becoming endemic—meaning that it will continue to evolve, circulate, and cause outbreaks. Therefore, societies across the region must also adapt as COVID-19 continues to affect individuals, communities, businesses, and governments. Although not the entire solution to stopping the spread of COVID-19, vaccination will continue to play a crucial role in the future of fighting the pandemic.
Our interviews with commissioners and advisors to the Asia-Pacific Hub revealed a triple challenge in the Asia Pacific, namely (1) pursuing equitable distribution of vaccines, (2) ensuring that rollout programs successfully deliver shots into arms, and (3) developing agile manufacturing capacity to produce essential drugs and vaccines.
“As variants keep emerging, there are three ways that vaccines may be needed in the future: vaccination need will be ongoing and frequent, vaccination will be needed annually, or vaccination will produce permanent immunity. These possibilities highlight the need for regional research and development, manufacturing, and supply chains to improve.”
This report draws on our interviews with regional experts, whose insights are referenced throughout, and additional research to discuss how vaccination in the Asia Pacific has affected resilience during the pandemic and what is needed for future resilience. The subsequent sections describe each aspect of the triple challenge in terms of the issues the region faces, actions countries have taken, and what is still needed in the Asia Pacific to promote future resilience.
The Asia Pacific is a diverse region, which is reflected in the large disparities in full vaccination coverage. This section explores the factors affecting vaccine access and equitable distribution in the Asia Pacific compared with other regions of the world as well as among countries within the region.
The Regional Challenge: A Disadvantage in the Global Vaccine Market
As many of our contributors emphasized, vaccination is crucial for the resilience of communities, businesses, and countries in the Asia Pacific. Full vaccination reduces the burdens of illness on families, communities, businesses, and health systems; moreover, the WHO advises that population immunity against COVID-19 is best achieved through vaccination, not through exposure to the pathogen that causes the disease.[4]
At the time of writing, some vaccines are more available than others in the region. Three vaccines, those by AstraZeneca, Sinovac, and Sinopharm, appear to be the most widely used. The Oxford–AstraZeneca vaccine is currently the only one supplied at cost rather than for profit, making it the most affordable and prevalent vaccine for countries in the Asia Pacific. It is offered in nearly all countries in the region except China.
Figure 5 shows where particular vaccines are currently offered. China focused on middle- and lower-income countries, including many in Southeast Asia, for early deliveries of its Sinopharm and Sinovac vaccines. This has made the country a major supplier of COVID-19 vaccines in the region, resulting from decisions by both the United States and India to prioritize vaccine production for their domestic populations to address severe waves of infections within their own borders.[5] However, the Chinese-developed vaccines are not used in South Korea, Japan, or Taiwan.[6] At the time of writing, the Pfizer, Moderna, and Johnson & Johnson vaccines account for a relatively small percentage of vaccines administered in the region.[7]
Figure 5. Where selected vaccines are used.
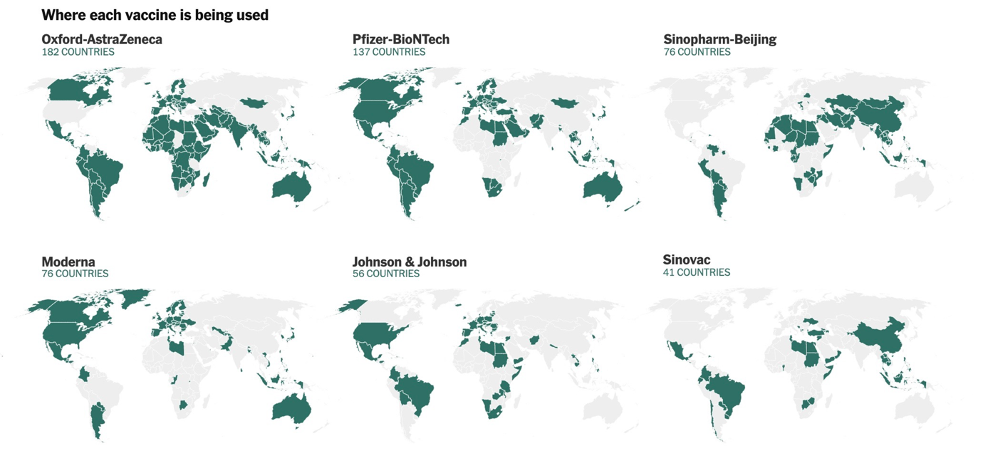
Source: The New York Times
Another aspect of the vaccination challenge for Asia-Pacific countries is the cost of vaccines. According to UNICEF’s vaccine dashboard, the current price range for COVID-19 vaccines is US$2–US$37 per dose.[8] Additional factors make some vaccines more expensive to administer than others. For example, in contrast to the AstraZeneca vaccine, which is sold at cost (US$2.15–US$5.25 per dose) and requires only standard refrigeration for storage, the messenger RNA (mRNA) vaccines by Pfizer–BioNTech (US$19.50 per dose for the first 100 million doses) and Moderna (US$25–US$37 per dose) must be stored and transported at very low temperatures, incurring additional costs. Consequently, fewer countries in the region can access them.
However, vaccine cost does not fully explain the challenge of vaccine access in the region. Without a global agreement on how vaccine distribution would be achieved equitably—or a consensus on what factors define greatest need—vaccine procurement during the pandemic has been left to competitive market forces. As such, global vaccine supply has been shaped by agreements between manufacturers and wealthy countries to purchase vaccines during their development. As our contributors noted, many countries in the Asia Pacific did not have sufficient capacity to plan, negotiate, or execute such agreements with vaccine manufacturers.
For example, both the United States and the United Kingdom struck early agreements with vaccine developers, namely Pfizer–BioNTech and Moderna in the United States and Oxford–AstraZeneca in the United Kingdom, to purchase vaccines in mid to late 2020.[9] By March 2021, the United States and United Kingdom had reserved enough doses to fully inoculate approximately 200% and 340% of their populations, respectively, and Canada purchased even more.[10] Meanwhile, the European Union reached purchase agreements with manufacturers including AstraZeneca, Pfizer–BioNTech, CureVac, and Moderna. These large purchase agreements that now seem excessive, one contributor noted, were entered into because the effectiveness of the vaccines was unknown at the time.
In the Asia Pacific, India had purchased enough AstraZeneca and Novavax doses by early March 2021 to cover 85% of its population. The Serum Institute of India obtained a contract in early 2021 to manufacture the Oxford–AstraZeneca vaccine for use within and outside the country. Singapore also purchased vaccines early, making it the first Asian country to receive the Pfizer–BioNTech vaccine in December 2020.[11] By contrast, authorities in places such as Australia and Taiwan did not purchase sufficient vaccines for their populations in 2020, delaying vaccine rollout to later in 2021.
Many of our interviews noted that although these agreements accelerated the development of life-saving vaccines, they resulted in only a few countries and regions having early access to them. As a result, countries that did not start purchasing vaccines early have had to wait for countries that reserved early supply to vaccinate their populations first. Relatedly, COVAX, the initiative co-led by Gavi, the Coalition for Epidemic Preparedness Innovations, and the WHO to finance vaccine distribution to lower-income countries, faced initial shortages in early 2021.[12]
“Competition may work for innovation, but it affects sharing.”
Moreover, export restrictions delayed vaccine rollout in the Asia Pacific in early 2021. Among the first of such restrictions was the result of a commercial dispute within the European Union with AstraZeneca. Consequently, a shipment of vaccines to Australia was blocked in March 2021.[13] In addition, although India assumed a key role in producing and exporting the Oxford–AstraZeneca vaccine, a devastating wave of domestic COVID-19 infections compelled the country to restrict vaccine exports in April 2021; India only resumed small-scale vaccine exports to its neighbors in October.[14] Similarly, in Thailand, delayed manufacturing slowed expected vaccination progress in Malaysia, the Philippines, and Taiwan in mid-2021.[15]
Although the global supply of vaccines is now stabilizing and increasing—exports from the EU are recovering, and India has now administered over one billion doses domesticly and is planning to increase exports—the imposition of these export restrictions in 2021 highlights the understandable desire of manufacturing countries to vaccinate their own populations before exporting to other regions. The balance between domestic and international commitments is difficult to strike in an emergency, and our contributors were concerned that the world does not yet have a solution for this balance. If not addressed, similar situations are likely to reoccur in future emergencies.
Facing limited access to vaccines, countries in the Asia Pacific are utilizing ad hoc, flexible mechanisms to receive donated, borrowed, and resold vaccines from countries within and outside the region that have excess supplies.
Japan has donated millions of vaccines to Asian countries including Indonesia, Malaysia, Philippines, Thailand, Taiwan, and Vietnam, and it has pledged 11.3 million doses to countries in Asia and the Pacific islands through the COVAX Facility.[16] Similarly, the Hong Kong government has announced that it is donating 7.5 million surplus doses of the AstraZeneca vaccine to COVAX.[17] China has also donated Sinopharm and Sinovac vaccines to Asia-Pacific countries, which have received 75% of China’s total overseas vaccine donations.[18] Further afield, the United Kingdom has donated vaccines to the Philippines, Laos, Cambodia, Indonesia, and Malaysia.[19]
Some countries are striking bilateral “swap” deals to utilize extra doses and achieve mutual benefits for both parties.[20] For example, an Australia–Singapore swap in August this year enabled Australia to access 500,000 doses, and Australia agreed to return the doses in December, when Singapore will be rolling out booster shots. Similar arrangements have been recorded between Australia and the United Kingdom as well as between South Korea and Israel.
Asia-Pacific countries are also purchasing excess vaccines from other countries, especially when local infections spike and vaccines are urgently needed. For example, Australia purchased excess Pfizer vaccines from Poland.[21] Similarly, New Zealand has purchased vaccines from Denmark and Spain.[22]
In many cases, these trading and donation mechanisms are examples of “vaccine diplomacy,” which relies on governmental cooperation and diplomatic capacity as well as favorable relationships between countries. This means that the flexible trading mechanisms developing in the Asia Pacific have geopolitical dimensions.[23]
For example, despite being a wealthy economy, Taiwan encountered difficulties early in procuring vaccines but received donated doses from countries including the United States, Japan, the Czech Republic, Poland, and Lithuania.[24] In many cases, these donations were acts of reciprocal goodwill toward Taiwan after the island donated face masks and other protective equipment to these and other countries in 2020.
In addition, Cambodia’s high vaccine coverage is partially attributable to its close relationship with China, whose Sinovac and Sinopharm vaccines accounted for 27 million of the 30 million doses Cambodia had bought or received as donations by early September 2021.[25]
Equitable vaccine distribution is the foundation of resilience in the Asia Pacific as COVID-19 becomes endemic.
Multilateral cooperation through COVAX as an innovative global mechanism is critical to future global resilience. Beyond COVAX, societies in the Asia Pacific must continue to explore their own approaches to interregional and intraregional vaccine procurement and distribution. This can include public–private collaboration to finance vaccine acquisition and administration. These mechanisms can help ensure that all societies in the region can access sufficient vaccines to meet vaccination targets and continue to access them on an ongoing basis as COVID-19 becomes endemic.
“The biggest issue now is equity. The equity problem will last for the next 18 months at least, especially as countries start to use booster shots. Advanced economies will continue to put pressure on demand, making inequity last for some time.”
However, any mechanisms for trading vaccines should complement, not replace, direct purchasing from manufacturers and distribution efforts through COVAX to achieve equitable vaccine access.
For example, bilateral vaccine swaps are beneficial because they can quickly relocate excess vaccines to countries that can immediately use them, rather than placing pressure on manufacturers to accelerate production. But because these ad hoc mechanisms rely on countries’ diplomatic and financial capacity to negotiate for excess doses as well as the infrastructure to rapidly administer vaccines nearing expiration, vaccine swapping and trading can increase efficiency, but not necessarily equity, in distribution.
The European Union is an example of a regional organization successfully helping its members acquire and distribute vaccines; the strategy enabled the small country of Portugal fully vaccinate over 80% of its population. As our contributors noted, the equitable distribution of vaccines in the Asia Pacific will benefit from a regional strategy with more engagement and coordination among all members. For example, ASEAN, which consists of many small and medium-size countries, could play a major role in this regard.
A key component to future resilience in the Asia-Pacific region is not only the distribution of vaccines to countries but the rollout of vaccination programs within them. This section summarizes both the challenges and necessary actions identified to improve the administration of vaccines in the region.
“Many countries are experiencing both vaccine shortages and vaccine hesitancy.”
Even with a sufficient and equitable vaccine supply, the swift rollout of vaccination programs depends on adequate infrastructure and public confidence, as many of our contributors explained. Many developing countries in the region have limited public health infrastructure that hinders vaccine rollout, including challenges in transporting and delivering vaccines, preparing proper public health facilities, and training and certifying the workforce needed for vaccine administration.[26] In addition, vaccine hesitancy is a common concern observed in the region. Together, these challenges are the greatest hindrance to Asia-Pacific societies finding a path toward resilience.
Our contributors emphasized that vaccine rollout must achieve “last-mile delivery” (i.e., “getting shots in arms”). A vaccination strategy that targets and quickly rolls out doses to cover all populations, including the most vulnerable, is critical. This requires a strong public health infrastructure program. Yet building and operating such an infrastructure remains challenging for developing countries, including those in the Asia Pacific.
Diverse socioeconomic conditions among and within countries have contributed to difficulties in vaccine rollout. For example, a government task force evaluated Indonesia’s vaccination drives to be “manageable” in urban areas but challenging in rural areas scattered among the country’s numerous islands, where power, transportation, and health workers are limited.[27] Similar challenges have been identified in the mountainous regions of other countries of Southeast Asia. The hurdle of distributing vaccines to remote areas becomes more challenging when vaccines require cold chain networks for storage and transport.[28] In addition, a two-dose administration schedule is needed for most COVID-19 vaccines, adding another challenge to delivery.
One country with a successful vaccine rollout in the region is Cambodia; by August 2021, it had fully vaccinated over 80% of its adult population, and its economy and society have been positioning to reopen ahead of its neighbors.[29] Swift vaccination was achieved through a strategy of sourcing multiple vaccine brands and a location-based rollout rather than age tiering, but the strategy involved strict measures, such as mandatory vaccination, enforced by the country’s strong national government without an organized opposition.[30]
People’s willingness to be vaccinated is another factor influencing the speed and scope of vaccine rollout. Vaccine hesitancy is not an issue exclusive to developed countries. In fact, refusal to accept vaccination despite the availability of vaccines was noted as one of the top 10 global health threats even before the pandemic; this hesitancy will hinder vaccination progress around the globe, including the Asia Pacific, if not properly and promptly addressed.[31]
According to our contributors, vaccine hesitancy in the region cannot be attributed to a single factor; rather, it is likely the result of a range of factors:
First, some people in the region feel unsure about vaccine science and new technologies. According to a survey in the Philippines, concerns about the side effects, effectiveness, and safety of vaccines were considered a barrier to vaccination.[32] In Australia, a lack of “health literacy” has been identified to impact confidence in vaccines.[33]
Second, even for those willing to be vaccinated, the perceived efficacy of vaccines is a critical factor in accepting vaccination. “Vaccine shoppers” wait to access vaccines perceived to be more effective and trustworthy than currently available brands.[34] Selective vaccine hesitancy toward Chinese vaccines has been observed in Malaysia, Thailand, and Vietnam, where people have voiced concerns about the developers’ lack of transparency.[35] In Australia, where vaccine rollout was already delayed by slow policy decisions on vaccine procurement, people have waited for the Pfizer vaccine instead of getting the available AstraZeneca vaccine after confusing government messaging.[36]
Finally, it is well understood that vaccine misinformation circulating online hinders government vaccination plans in many countries. For example, religious micro-influencers in Indonesia fuel public ambivalence toward the government’s vaccination campaign by spreading conspiracies about vaccines in a religious context.[37] Efforts by national governments to reduce the spread of misinformation on social media platforms risk public criticism where trust in governments is low.[38]
Our regional contributors noted that, although the challenges surrounding vaccine rollout vary at the local level, they have considerable regional and global impacts. Unvaccinated populations remain vulnerable to infection, and these vulnerabilities throw cold water on regional reconnection initiatives, as demonstrated by the repeated failures to establish a travel bubble between Hong Kong and Singapore and to maintain a travel agreement between Australia and New Zealand.[39] Continued border closures and other travel restrictions, such as mandatory quarantines, trigger frustration among people, further erode public trust in governments, and place regional and global supply chains under additional pressure.
The anticipated delays in vaccine rollout also mean that the emergence of a new SARS-Cov-2 variant would be devastating to current global vaccination efforts. This makes the possibility of a “return to normal” even more distant for many countries in the Asia-Pacific region and beyond. To this end, our experts noted that a regional vaccination strategy will become important over time.
As our contributors experts noted, resilience relies on vaccines that arrive in the region being administered into people’s arms as quickly as possible to avoid losing the race to end the pandemic at the last mile.
To this end, wealthy countries in the region are helping their developing neighbors. For example, Australia has partnered with UNICEF to enhance Vietnam’s COVID-19 response by supplying cold chain storage facilities and funding health worker training. Both initiatives are crucial for vaccine rollout in the remote provinces of Vietnam.[40] The Asia Pacific Vaccine Access Facility (APVAX) initiative launched by the Asian Development Bank—aiming to provide developing Asia with financial resources for vaccine procurement, delivery, and administration as well as community outreach and surveillance—is another welcome move that shows the potential for regional collaboration on vaccine rollout.[41]
“People understand the economic importance of keeping COVID-19 cases low; the economy is starting to recover, and we cannot afford another lockdown.”
National and local vaccination policies and programs as well as effective communication are crucial for vaccine rollout and uptake. For example, throughout 2020 and 2021, Taiwan’s Central Epidemic Command Center has been the government agency providing updates and information on COVID-19 developments and policies.[42] In addition, Singapore has demonstrated how public communication and trust building can facilitate vaccine rollout in the country (see Box below).
Without comprehensive and swift vaccine rollouts, all stakeholders in the Asia Pacific are at a continuing disadvantage, undermining regional resilience.
“Capacity to roll out vaccines is an important infrastructure.”
According to our contributors, regional experiences have shown that intraregional cooperation at both national and local levels is imperative to support vaccine rollouts, especially in developing countries where public health infrastructure can be inadequate. A regional vaccination strategy can identify how cooperation can support equity in both distribution and rollout. In addition, businesses can mobilize their regional networks to complement government-led efforts to deliver vaccines to communities, as they did to acquire and deliver medical equipment to India during its severe wave of infections in early 2021.[43]
Targeted public communication is crucial for translating scientific knowledge into lay terms, understanding public concerns, and combating misinformation. A whole-of-society approach is needed in which governments, businesses, academics, health care providers, and civil society all contribute to building trust and consensus on vaccination to ensure the last-mile delivery of vaccines.
Box: Addressing Vaccine Hesitancy and Trust in Government: A Comparison of Hong Kong and Singapore
Singapore and Hong Kong, arguably two of the most agile societies in the region, demonstrated good track records of preventive measures and preparedness early in the COVID-19 pandemic.[44] Both places secured enough vaccines for their entire populations in 2020 thanks to early planning and reliance on the expert community. However, the two jurisdictions have experienced varied trajectories of vaccination rollout that have led them to very different positions in the current battle against the COVID-19 pandemic.
In surveys conducted early in the pandemic in Hong Kong, a lack of confidence in vaccine technology, manufacturers, and production countries were all cited as reasons for vaccine hesitancy.[45] Moreover, a lack of trust in the Hong Kong government among young people contributed to vaccine hesitancy, despite months of intensive government-led vaccination campaigns.[46]
Later in the vaccination campaign, businesses and other social sectors joined in to provide more incentives, including paid leave and a city-wide lottery with a luxury apartment as the top prize for vaccinated people.[47] The government also introduced a walk-in scheme for senior residents to get vaccinated without an appointment. These facilitatory measures all have been instrumental in increasing uptake.
Yet as our experts identified, barriers to vaccination remain in the city. Older residents are asked to seek advice on vaccination from their family doctors, who are widely trusted in Hong Kong. However, the government has not provided clear guidance to medical professionals for advising vaccination to their patients. The resulting caution exercised by family doctors, especially for patients with underlying conditions, led to many senior people in Hong Kong refusing the vaccine.[48] Therefore, vaccination coverage among people aged 80 or older—the most vulnerable group in the pandemic—has only recently passed 10%.[49]
Given its stagnating vaccination program, the Hong Kong government has maintained a “zero-case” strategy since strict border control started in February 2020, and the reopening of borders, including with Mainland China, is not yet possible.[50] Consequently, Hong Kong is in a stalemate with COVID-19, with its economy suffering and community locked in a mood of frustration.[51]
By contrast, Singapore pursued a swift vaccination process, throughout which public trust in the government has been crucial. The Singaporean government conducted a widespread public communication campaign promoting vaccination, mobilized community volunteers, and collaborated with social groups to maximize social engagement.[52]
Specifically, Singapore’s vaccination strategy has targeted elderly people through multimedia promotion, community engagement, and professional persuasion to ease their concerns, with support from various grassroots associations to arrange one-stop vaccination appointments at home or help older people reach vaccination centers.[53]
In addition, an exit strategy was planned months ahead, and the government signaled that it would follow this strategy in June 2021.[54] The roadmap toward “COVID resilience” was presented as an easing of various control measures in stages conditioned on sustained high vaccination coverage.[55]
So far, Singaporeans have generally accepted this roadmap and have moved forward even as the number of COVID-19 cases remains in flux. With its high vaccination coverage, simplified health care protocol, and rapid testing capacity, Singapore is exploring a pathway toward living with endemic COVID-19 and building resilience by adapting and innovating its vaccination strategy.[56]
As the previous sections have shown, the distribution and rollout of vaccines during the pandemic have been significant challenges in the Asia Pacific. Despite these obstacles, our interviews revealed that countries in the region have adapted by pursuing manufacturing agreements, laying the foundations for future resilience.
One of the most important issues identified by our contributors in vaccine distribution and rollout in the Asia Pacific has been the geographical concentration of manufacturers producing vaccines in a small group of countries.[57] China and India—both regional manufacturers—are among the top vaccine producers in the world (Figure 6). For example, China has become the largest producer of finished vaccines during the pandemic, and it exported over 1 billion doses to 109 countries and regions over 10 months.[58]
Figure 6. Countries Producing and Exporting COVID-19 Vaccines.
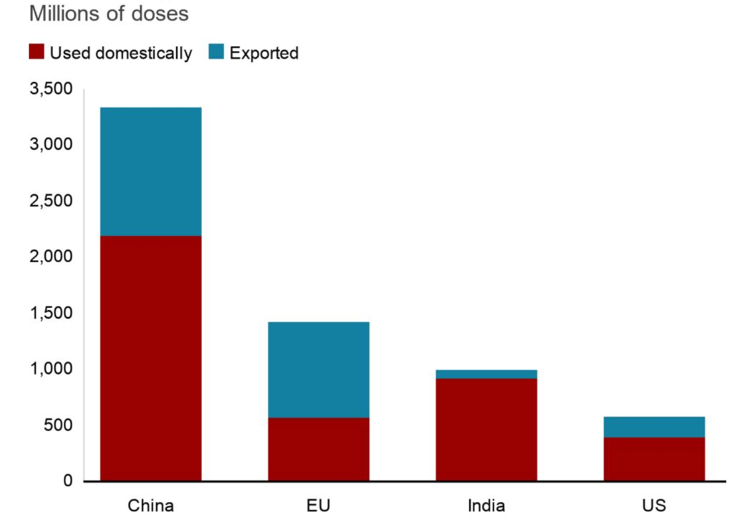
Source: BBC, Our World in Data, Airfinity and Indian government. The domestic numbers are as of October 6, and export numbers are as of October 8, 2021.
“Global agencies have little power under current arrangements. Without an agreement, countries can dominate the process of manufacturing and distribution.”
However, as noted earlier, with no global agreement on the equitable distribution of essential drugs and vaccines during a health emergency, vaccine manufacturing, procurement, and distribution was left open to market and political processes.[59] Concentrated production meant that countries with manufacturing capacity, such as the United States and United Kingdom, could procure vaccines first. Some countries and regions also imposed export restrictions to address their urgent domestic needs. Consequently, there was little coordinated thought in the region, or globally, as to how to support other countries.
As a result, calls have emerged globally to improve vaccine manufacturing and distribution by waiving intellectual property rights, declaring vaccines to be global “common” or “public” goods, and establishing a global treaty on emergency drug distribution.[60] However, our contributors argued that these mechanisms take time and do not address the short-term vaccine supply issues or resolve inequitable distribution in the Asia Pacific.
Our contributors pointed out that agile manufacturing—the ability of countries to expand drug and vaccine manufacturing quickly and effectively to provide “surge capacity” for exports in a health emergency—is a remarkable development in the region.
Governments and businesses in the Asia Pacific have acted quickly to secure opportunities to establish contract development and manufacturing organizations (CDMOs) in their jurisdictions to manufacture and distribute vaccines for pharmaceutical companies (Figure 7).[61] These CDMO agreements are beneficial to both regional and global resilience because they have been established in “agile” countries with smaller populations (than, for example, the United States or India) that can more flexibly mobilize to manufacture and export COVID-19 vaccines. Therefore, such “agile” manufacturing countries have a greater chance of addressing surge capacity during health emergencies.
Figure 7. Locations of COVID-19 vaccine manufacturers as of September 6, 2021.
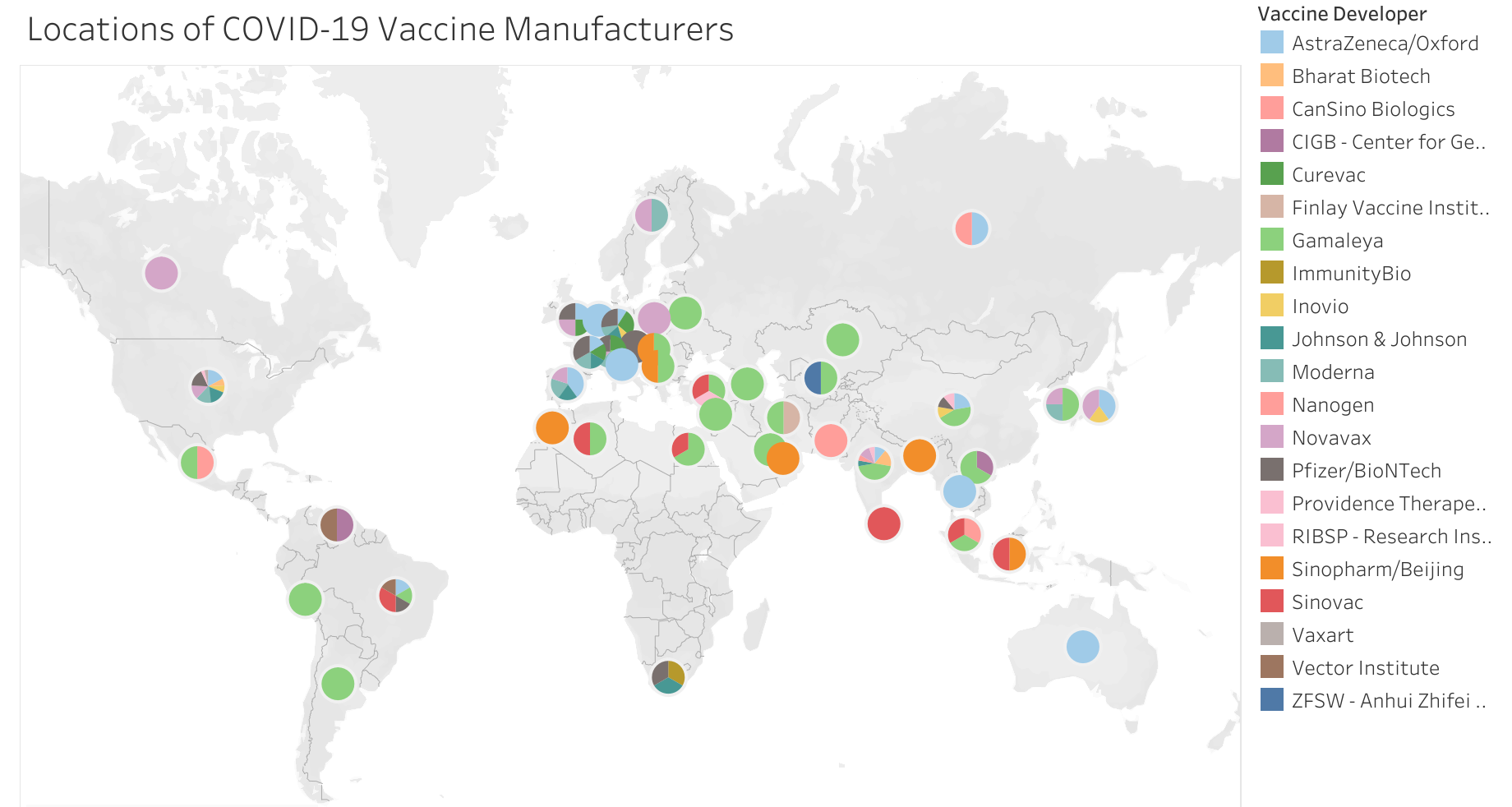
Source: Graduate Institute Geneva, sourced 10 October 2021.
For example, at the time of writing, South Korea has emerged as a major vaccine manufacturing site in the region, with SK Bioscience producing AstraZeneca and Novavax vaccines and Samsung Biologics starting production of Moderna vaccines soon. In addition, other potential vaccine manufacturing deals have been reported.[62] Japan has also reached out to global vaccine makers, with Daiichi Sankyo and other pharmaceutical firms manufacturing the AstraZeneca vaccine, and Takeda starting manufacture and distribution of the Novavax vaccine in early 2022.[63] Further, BioNTech announced the construction of a new mRNA vaccine manufacturing facility in Singapore, to be operational in 2023.[64]
“There is great value in being agile and a trusted partner during a pandemic.”
Other countries in the region are also seeking to build manufacturing capacity. In Thailand, drug maker Siam Bioscience has served as the only producer of AstraZeneca’s vaccine in Southeast Asia.[65] In Indonesia, local pharmaceutical company Bio Farma has been licensed to produce the Chinese developer Sinovac Biotech’s vaccines for domestic use, and the Indonesian government has been in talks with the WHO to find opportunities for transforming Indonesia into an advanced global vaccine manufacturing hub.[66]
However, any CDMO arrangements established during an emergency must also consider their financial viability when there is no pandemic. COVID vaccine manufacturing requires not only a sizable and sustainable output market but also the inputs necessary to support a large, intensive, and specialized investment. Some countries in the region have attempted but failed to reach manufacturing agreements with vaccine developers. For instance, Taiwan is known for its high-technology manufacturing sector, but still lacked the qualifications to reach an agreement with AstraZeneca to produce COVID-19 vaccines.[67]
According to our contributors, harmonized national regulatory frameworks for drug development and use are critical for both the quality and agility of this new manufacturing capacity. First, companies seeking to manufacture currently available vaccines must meet specific national standards for drug quality, efficacy, and production output. Further, importing countries must either accept the regulatory standards of other countries to quickly access their newly approved vaccines or else provide additional regulatory approval rapidly. Although the WHO has guidelines for vaccine developers to apply for the emergency use of their vaccines, drugs, and therapeutics, it has no specific recommendations for governments in formulating their own national emergency approval regulations. In addition, although many members in the region have successfully invested in domestic public health institutions, some less-developed countries in the Asia Pacific still lack the capacity to oversee or regulate medical products, including vaccines, or accelerate emergency use approval during a pandemic.
Fragmented national regulations and disparities in regulatory capacity are already creating additional challenges in the region. The emergency approval of vaccines in the current pandemic has revealed inconsistencies in the international regulatory landscape. Concerns about emergency approval processes have delayed the introduction of vaccines in some countries, which are requiring full regulatory procedures to be finalized prior to rollout. For example,
Japan’s requirements for domestic clinical trials delayed its approval of the Pfizer–BioNTech vaccine for several months, resulting in slow vaccine rollout.[68]
Seeking full regulatory approval for vaccines rather than emergency use approval also delayed New Zealand’s vaccination program, forcing the country to continue relying on border controls and domestic containment strategies to manage outbreaks.[69]
Of the lower- and middle-income countries in the region, few have functional, mature national regulatory authorities as assessed by the WHO, potentially undermining the reliability of a regional manufacturing network.[70]
As COVID-19 becomes endemic, demand for vaccines will persist, particularly as booster shots become more widely administered. The regional vaccine manufacturing network must be expanded to meet this projected demand. Without well-recognized regulatory and quality assurance mechanisms, efficient approval and distribution of vaccines across the region will be difficult to achieve. In addition, new and modified vaccines will continue to be developed in the Asia Pacific and elsewhere to address emerging variants of the virus. The current inconsistencies in regulatory mechanisms may affect public confidence in new vaccines and countries’ ability to swiftly acquire and administer them.
“We may need to rethink global supply chains to develop surge capacity for emergencies in countries where there is trust in the process and they have smaller populations which can be vaccinated quickly.”
The need for both agility and quality in regional manufacturing was a distinctive theme in our interviews with our contributors. Although some argue that not all vaccines are easily licensed for manufacturing, the experience in the Asia Pacific shows that licensing to manufacture is possible in regions with agile and high-quality manufacturing capacity.[71] The policymaking and regulatory institutions surrounding manufacturing in the region are critical for future resilience. Fortunately, many countries in the region have science, technology, and innovation policies that have sponsored R&D and new manufacturing activities.
These policies can support the development of agile manufacturing capacity in the region, with a specific focus on surge capacity during health emergencies. Provided that this agile capacity can be developed, our contributors stressed that harmonized regulations within the region will be needed to enhance long-term resilience in future health emergencies.
Using the example of vaccines, this report shows that the concept of resilience can be used as a framework to better understand the ways in which systems in the Asia Pacific have adapted, evolved, and innovated.
Although vaccine equity has been a challenge, countries have taken action to develop flexible trading mechanisms that are complementary to global and market mechanisms.
To address weaknesses in public health infrastructure, countries are helping their neighbors achieve the last-mile delivery of vaccines.
Finally, countries in the Asia Pacific are developing agile manufacturing capacity for future health emergencies through CDMO opportunities.
Although vaccines are a key to entering the post-pandemic world, the solution for preventing another pandemic and the crises that ensue lies in governments, businesses, academics, and civil society committing to building systems that can adapt, evolve, and innovate more quickly and coherently in the face of these new challenges.
Therefore, the Asia-Pacific Hub of the Resilience Commission will continue convening and researching regional issues related to resilience. The Hub and the Miller Center of the University of Virginia along with the American and European hubs of the Resilience Commission convened an online, global event on October 26, 2021, to discuss the findings and recommendations of this report: “Vaccination and beyond: Lessons for ending the pandemic from the Asia-Pacific region, Europe, and the U.S.” Thereafter, the Hub’s research will focus on developing further regional strategies for enhancing resilience, including multilateral cooperation, cross-sector collaboration, and innovative local governance.
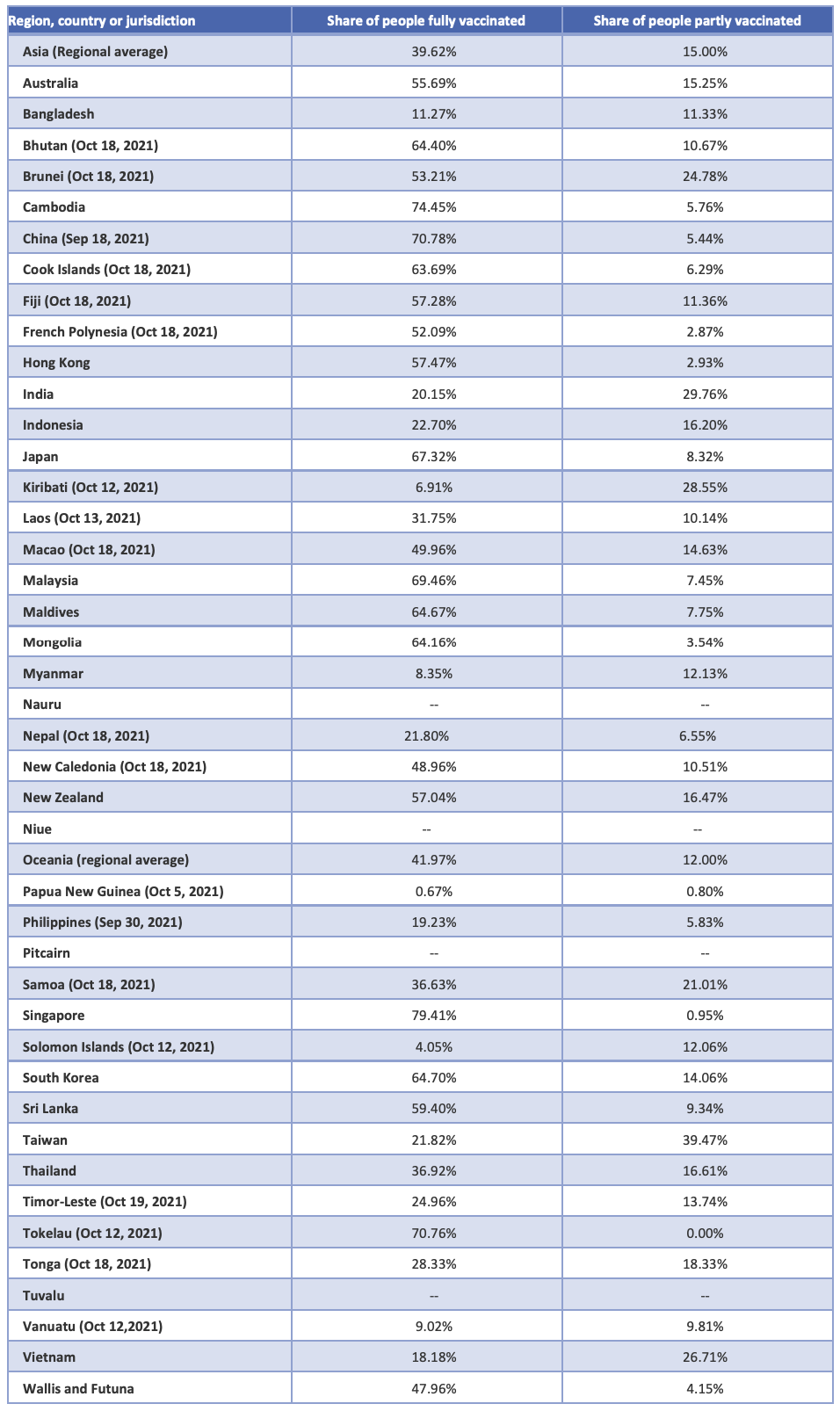
This report was released in October 2021. During the drafting of the report, it was important to use consistent data in what proved to be a fast-moving data environment. No official global database for COVID-19 deaths, cases, or vaccination coverage has been compiled. However, some sites have compiled data during the pandemic across countries.
In this report and appendix, we use available data for vaccination percentages recorded as of October 17, 2021, or the closest date available, from the Our World in Data website, which compiles comparative data on vaccinations, cases, deaths, and hospitalizations worldwide into one database.[72]
This report has included the countries and jurisdictions within the geographic boundaries of the WHO regions for South East Asia and Western Pacific.
Data are available for many, but not all, of these countries and jurisdictions in Our World in Data. This report therefore uses Our World in Data as the “best available,” rather than a comprehensive, source of comparative data.
Table. Available data on share of people vaccinated by dose, October 17, 2021, or closest available date.[73]
1 See, for example, Rohit Gupta and Stephanie R Morain. “Ethical allocation of future COVID-19 vaccines.” Journal of Medical Ethics 47, no. 3 (2021): 137-141; Rafael Dal-Ré and Victoria Camps. “Who should be vaccinated against COVID-19 first?.” Medicina Clínica (English Edition) 156, no. 4 (2021): 177-179.
2 Given the fast-moving data environment, country vaccination data used in this report has been sourced from Our World in Data, and is replicated in the Appendix.
3 “How much will vaccine inequity cost?” The Economist Intelligence Unit, August 25, 2021, https://www.eiu.com/n/delayed-vaccination-timelines-will-cost-the-global-economy-us2-3trn/.
4 “Coronavirus disease (COVID-19): Herd immunity, lockdowns and COVID-19,” World Health Organization, December 31, 2020, https://www.who.int/news-room/q-a-detail/herd-immunity-lockdowns-and-covid-19.
5 Lionel Guetta-Jeanrenaud, Niclas Poitiers, and Reinhilde Veugelers, “A world divided: global vaccine trade and production,” Bruegel, July 20, 2021, https://www.bruegel.org/2021/07/a-world-divided-global-vaccine-trade-and-production/.
6 Michael Leigh, “Vaccine diplomacy: soft power lessons from China and Russia?” Bruegel, April 27, 2021, https://www.bruegel.org/2021/04/vaccine-diplomacy-soft-power-lessons-from-china-and-russia/.
7 “Production, politics and propaganda: How Beijing has shaped the international COVID immunization drive,” Nikkei Asia, October 12, 2021, https://asia.nikkei.com/static/vdata/chinavaccine-1/.
8 “COVID-19 Vaccine Market Dashboard,” United Nations Children’s Fund, October 2021, https://www.unicef.org/supply/covid-19-vaccine-market-dashboard.
9 Chad P. Bown and Thomas J. Bollyky, “How COVID-19 vaccine supply chains emerged in the midst of a pandemic,” Peterson International Institute Working paper 21-12, August 2021, https://www.piie.com/publications/working-papers/how-covid-19-vaccine-supply-chains-emerged-midst-pandemic.
10 “Covid-19 deals tracker: 9.6 billion doses under contract,” Bloomberg, March 9, 2021, https://www.bloomberg.com/graphics/covid-vaccine-tracker-global-distribution/contracts-purchasing-agreements.html?sref=33gntO7X.
11 Gianna Gayle Amul, Michael Ang, Diya Kraybill, Suan Ee Ong, Joanne Yoong. “Responses to COVID-19 in Southeast Asia: Diverse paths and ongoing challenges,” Asian Economic Policy Review (2021), https://doi.org/10.1111/aepr.12362.
12 Seth Berkley. “COVAX: more than a beautiful idea,” The Lancet 398, no. 10298 (2021), https://doi.org/10.1016/S0140-6736(21)01544-0.
13 In March 2021, the Italian government blocked a shipment of approximately 250,000 doses of the AstraZeneca vaccine bound for Australia after the company failed to meet its contract commitments to the EU for vaccine production. The European Commission backed the decision of the Italian government. See “EU blocks export of AstraZeneca COVID-19 vaccines to Australia.” DW, March 4, 2021, https://www.dw.com/en/eu-blocks-export-of-astrazeneca-covid-19-vaccines-to-australia/a-56774362.
14 Sanjeev Miglani, “India resumes vaccine exports as domestic stocks build up – officials.” Reuters, October 13, 2021, https://www.reuters.com/world/india/india-resumes-vaccine-exports-domestic-stocks-build-up-officials-2021-10-13/.
15 Hannah Beech and Muktita Suhartono, “A mass inoculation campaign in Thailand stumbles amid a severe outbreak,” The New York Times, June 14, 2021, https://www.nytimes.com/2021/06/14/world/thailand-shortage.html.
16 “Japan to give millions more AstraZeneca shots to Asia,” Nikkei Asia, June 25, 2021, https://asia.nikkei.com/Spotlight/Coronavirus/COVID-vaccines/Japan-to-give-millions-more-AstraZeneca-shots-to-Asia.
17 Elizabeth Cheung and Tony Cheung, “Hong Kong to donate 7.5 million doses of AstraZeneca vaccine to developing countries,” South China Morning Post, October 12, 2021, https://www.scmp.com/news/hong-kong/health-environment/article/3152034/hong-kongs-quarantine-policy-big-reason-city-has.
18 “China COVID-19 Vaccine Tracker,” Bridge Consulting (Beijing), October 11, 2021, https://bridgebeijing.com/our-publications/our-publications-1/china-covid-19-vaccines-tracker/.
19 “UK begins donating millions of COVID-19 vaccines to countries overseas including the Philippines,” UK Government, July 28, 2021, https://www.gov.uk/government/news/uk-begins-donating-millions-of-covid-19-vaccines-to-countries-overseas-including-the-philippines.
20 Takashi Nakano, Fumi Matsumoto and Jun Suzuki, “From Australia to Thailand, vaccine swap deals help ease shortages,” Nikkei Asia, October 1, 2021, https://asia.nikkei.com/Spotlight/Coronavirus/COVID-vaccines/From-Australia-to-Thailand-vaccine-swap-deals-help-ease-shortages.
21 Paulina Duran, “Australia purchases Pfizer vaccines from Poland as COVID-19 infections spike,” Reuters, August 15, 2021, https://www.reuters.com/world/asia-pacific/australia-purchases-pfizer-vaccines-poland-covid-19-infections-spike-2021-08-14/.
22 Alexa Cook, “Jacinda Ardern announces second COVID-19 vaccine deal with Denmark, country set to receive extra 500,000 Pfizer vaccinations,” News Hub, September 12, 2021, https://www.newshub.co.nz/home/new-zealand/2021/09/arden-announces-second-covid-19-vaccine-deal-with-denmark-country-set-to-receive-extra-500-000-pfizer-vaccinations.html.
23 Coleman Beaty, “Japan and vaccine diplomacy,” Center for Strategic & International Studies, August 9, 2021, https://www.csis.org/blogs/new-perspectives-asia/japan-and-vaccine-diplomacy.
24 Thomas Shattuck, “Vaccines give Taiwan upper hand,” Taipei Times, July 30, 2021, https://www.taipeitimes.com/News/editorials/archives/2021/07/30/2003761702.
25 Sebastian Strangio, “What explains Cambodia’s COVID-19 vaccine distribution success?” The Diplomat, September 8, 2021, https://thediplomat.com/2021/09/what-explains-cambodias-covid-19-vaccine-distribution-success/.
26 Rebecca Forman, Soleil Shah, Patrick Jeurissen, Mark Jit, and Elias Mossialos. “COVID-19 vaccine challenges: What have we learned so far and what remains to be done?” Health Policy (Amsterdam) 125, no. 5 (2021): 553-67.
27 Jeffrey Hutton and Raul Dancel, “Logistics, regulatory bottlenecks loom as South-east Asia embarks on Covid-19 vaccine roll-out,” Strait Times, February 7, 2021, https://www.straitstimes.com/asia/se-asia/as-south-east-asia-embarks-on-covid-19-vaccine-rollout-logistics-regulatory-bottlenecks.
28 Cyn-Young Park, Kijin Kim, Matthias Helble, and Susann Roth, “Getting ready for the COVID-19 vaccine rollout,” ADB Policy Brief no. 166, February 2021, https://dx.doi.org/10.22617/BRF210071-2.
29 “Vaccination Nation: Unmasking Cambodia’s Vaccination Success,” Mekong Strategic Partners, August 2021, https://www.mekongstrategic.com/post/vaccination-nation.
30 Sam Macdonald, “Learning from Cambodia’s COVID-19 response,” Foreign Policy in Focus, September 22, 2021, https://fpif.org/learning-from-cambodias-covid-19-response/; Adrien Chorn and Jonathan Stromseth, “COVID-19 comes to Cambodia,” Brookings, May 19, 2021, https://www.brookings.edu/blog/order-from-chaos/2021/05/19/covid-19-comes-to-cambodia/.
31 “Ten threats to global health in 2019,” WHO, https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.
32 Alexandria Caple et al. “Interrogating COVID-19 vaccine hesitancy in the Philippines with a nationwide open-access online survey.” medRxiv (2021), https://doi.org/10.1101/2021.09.11.21263428.
33 Kirsten McCaffery et al. “Disparities in COVID-19 related knowledge, attitudes, beliefs and behaviours by health literacy.” medRxiv (2020), https://doi.org/10.17061/phrp30342012.
34 Fides A Del Castillo. “Changing the COVID-19 vaccine narrative to dispel vaccine hesitancy.” Journal of Public Health 43, no. 3 (2021), https://doi.org/10.1093/pubmed/fdab201.
35 Khairulanwar Zaini and Hoang Thi Ha, “Understanding the selective hesitancy towards Chinese vaccines in Southeast Asia,” ISEAS-Yusof Ishak Institute Perspective, September 1, 2021, https://www.iseas.edu.sg/articles-commentaries/iseas-perspective/2021-115-understanding-the-selective-hesitancy-towards-chinese-vaccines-in-southeast-asia-by-khairulanwar-zaini-and-hoang-thi-ha/; Chris Baraniuk. “What do we know about China’s covid-19 vaccines?” BMJ 373 (2021), https://doi.org/10.1136/bmj.n912.
36 Jamie Smyth, “Australia vaccine missteps keep it in the grip of Covid,” Financial Times, August 4, 2021, https://www.ft.com/content/3637a08d-f9cb-436e-9053-5563a29b1edd.
37 Yatun Sastramidjaja and Amirul Adli Rosli, “Tracking the swelling COVID-19 vaccine chatter on TikTok in Indonesia,” ISEAS-Yusof Ishak Institute Perspective, June 17, 2021, https://www.iseas.edu.sg/articles-commentaries/iseas-perspective/2020-82-tracking-the-swelling-covid-19-vaccine-chatter-on-tiktok-in-indonesia-by-yatun-sastramidjaja-and-amirul-adli-rosli/.
38 Akane Okutsu, Cliff Venzon and Erwida Maulia, “Indonesia and Philippines face persistent anti-vax hurdle,” Nikkei Asia, September 14, 2021, https://asia.nikkei.com/Spotlight/Asia-Insight/Indonesia-and-Philippines-face-persistent-anti-vax-hurdle.
39 Michael Smith and Emma Connors, “Hong Kong and Singapore part ways over borders,” Australia Financial Review, September 3, 2021, https://www.afr.com/world/asia/hong-kong-and-singapore-part-ways-over-borders-20210903-p58oil; “New Zealand, Australia travel bubble suspended for longer amid Delta outbreaks,” Reuters, September 17, 2021, https://www.reuters.com/world/asia-pacific/new-zealand-australia-travel-bubble-suspended-longer-amid-delta-outbreaks-2021-09-17/.
40 “Australia partners with Vietnam on vaccine rollout,” Minister for Foreign Affairs, Australia, August 26, 2021, https://www.foreignminister.gov.au/minister/marise-payne/media-release/australia-partners-vietnam-vaccine-rollout.
41 “$9 Billion ADB facility to help developing member countries access and distribute COVID-19 vaccines,” Asia Development Bank, December 11, 2020, https://www.adb.org/news/9-billion-adb-facility-help-members-access-and-distribute-covid-19-vaccines.
42 Chang-Chuan Chan and Chi-hsin Sally Chen, “The Taiwan model of COVID-19 control and its global implication,” Taiwan Strategists (2020) no. 6, https://www.pf.org.tw/files/6717/83781915-F11B-49C3-BC7A-16A6B191EBD3.
43 Pauline Yeung, “Networks for a Resilient Future: An Asian Perspective.” Asia Business Council, August 2021.
44 EK Yeoh et al. “Government response measures to COVID-19 – key policy lessons: From experiences of six middle/high income jurisdictions in the western Pacific region in the early stage of the pandemic,” Health System Impact Subgroup of the COVID-19 Social Science Working Group, World Health Organization, 2021.
45 Kailu Wang et al. “Change of willingness to accept COVID-19 vaccine and reasons of vaccine hesitancy of working people at different waves of local epidemic in Hong Kong, China: Repeated cross-sectional surveys.” Vaccines 9, no. 1 (2021): 62; Martin CS Wong et al. “Acceptance of the COVID-19 vaccine based on the health belief model: A population-based survey in Hong Kong.” Vaccine 39, no. 7 (2021): 1148-1156.
46 Timothy McLaughlin, “The place with surprisingly high vaccine hesitancy,” The Atlantic, April 1, 2021, https://www.theatlantic.com/international/archive/2021/04/hong-kong-trust-vaccine/618469/.
47 Sammy Heung, “Hong Kong developers dangle another free flat as vaccination incentive, with just one dose required this time,” South China Morning Post, July 30, 2021, https://www.scmp.com/news/hong-kong/hong-kong-economy/article/3143176/coronavirus-hong-kong-developers-dangle-another.
48 Bruce Einhorn, Thomas Shum, and Olivia Tam, “Hong Kong recovery imperilled by elderly saying no to vaccine,” Bloomberg, August 6, 2021, https://www.bloomberg.com/news/articles/2021-08-05/fearful-elderly-refusing-vaccines-threaten-hong-kong-s-recovery?sref=33gntO7X.
49 Hong Kong Vaccination Dashboard, HKSARG, https://www.covidvaccine.gov.hk/en/dashboard.
50 Tony Cheung and Gigi Choy, “Hong Kong leader lobbies Beijing for border reopening, asks for meeting of local, mainland medical experts,” South China Morning Post, September 8, 2021, https://www.scmp.com/news/hong-kong/health-environment/article/3147965/coronavirus-hong-kong-leader-lobbies-beijing.
51 John Power, “Vaccine hesitancy puts Asia’s ‘zero-Covid’ economies like Hong Kong, Australia in herd immunity stalemate,” South China Morning Post, May 23, 2021, https://www.scmp.com/week-asia/health-environment/article/3134408/vaccine-hesitancy-puts-asias-zero-covid-economies-hong.
52 Heather Humphries, “The role of public communications and engagement in a pandemic,” Ethos no. 22, June 29, 2021, https://www.csc.gov.sg/articles/the-role-of-public-communications-and-engagement-in-a-pandemic.
53 Stephanie Phang and Livia Yap, “Overcoming vaccine hesitancy, Singapore style,” Bloomberg, September 16, 2021, https://www.bloomberg.com/news/newsletters/2021-09-16/overcoming-vaccine-hesitancy-singapore-style?sref=33gntO7X.
54 Gan Kim Yong, Lawrence Wong and Ong Ye Kung, “Living normally, with Covid-19: Task force ministers on how Singapore is drawing road map for new normal,” Strait Times, June 24, 2021, https://www.straitstimes.com/opinion/living-normally-with-covid-19.
55 Wycliffe Enli Wei, Wei Keat Tan, Alex Richard Cook, Li Yang Hsu, Yik Ying Teo, and Vernon Jian Ming Lee. “Living with COVID-19: The road ahead.” Annals of the Academy of Medicine, Singapore 50, no. 8 (2021): 619-628.
56 Clara Chong, “S’pore’s Covid-19 strategy not a ‘flip-flop’, helps prevent massive number of deaths: Ong Ye Kung,” Strait Times, October 18, 2021, https://www.straitstimes.com/singapore/spores-covid-19-strategy-not-a-flip-flop-helps-prevent-massive-number-of-deaths-ong-ye.
57 Simon J. Evenett, Bernard Hoekman, Nadia Rocha, and Michele Ruta. “The Covid-19 vaccine production club: will value chains temper nationalism?” Policy Research Working Paper no. 9565 (2021). World Bank, Washington, DC., https://openknowledge.worldbank.org/handle/10986/35244.
58 Wanyuan Song, “Covid-19 vaccines: Has China made more than other countries combined?” BBC, October 10, 2021, https://www.bbc.com/news/58808889. Anna Nishino, “China’s global vaccine gambit: Production, politics, and propaganda,” Nikkei Asia, October 12, 2021, https://asia.nikkei.com/static/vdata/chinavaccine-1/.
59 For instance, China’s COVID-19 diplomacy featuring the provision of medical aid and vaccines has been subject to discussion; see ChinaPower Team, “Is China’s Covid-19 Diplomacy Succeeding?” Center for Strategic & International Studies, updated September 30, 2021, https://chinapower.csis.org/china-covid-medical-vaccine-diplomacy/.
60 “COVID-19: Make it the Last Pandemic,” Independent Panel for Pandemic Preparedness & Response, May 2021, https://theindependentpanel.org/mainreport/; Gordon Brown and Daniel Susskind. “International cooperation during the COVID-19 pandemic.” Oxford Review of Economic Policy 36, no. Supplement_1 (2020): S64-S76; Marc-Alain Widdowson, “The unfair distribution of vaccines: An embarrassing Deja-vu,” Institute of Tropical Medicine, January 25, 2021, https://www.itg.be/E/Article/vaccinongelijkheid-deja-vu.
61 Chad P. Bown and Thomas J. Bollyky, “How COVID-19 vaccine supply chains emerged in the midst of a pandemic,” Peterson International Institute Working paper 21-12, August 2021, https://www.piie.com/publications/working-papers/how-covid-19-vaccine-supply-chains-emerged-midst-pandemic; “COVID-19 Vaccine Manufacturing Agreements,” Global Health Centre, Graduate Institute Geneva, 2021, https://www.knowledgeportalia.org/covid19-vaccine-manufacturing.
62 “Janssen visits GC Pharma plant to spark a consignment speculation,” Pulse News Korea, September 29, 2021, https://pulsenews.co.kr/view.php?year=2021&no=926038.
63 “Japanese drugmaker starts AstraZeneca vaccine production,” The Japan Times, March 12, 2021, https://www.japantimes.co.jp/news/2021/03/12/national/astrazeneca-vaccine-japan/; “Japan to purchase 150 mln doses of Takeda-produced Novavax vaccines – drugmaker,” Reuters, September 7, 2021, https://www.reuters.com/business/healthcare-pharmaceuticals/japan-purchase-150-mln-doses-takeda-produced-novavax-vaccines-drugmaker-2021-09-07/.
64 Ludwig Burger and Caroline Copley, “BioNTech to build mRNA vaccine manufacturing site in Singapore,” Reuters, May 10, 2021, https://www.reuters.com/business/healthcare-pharmaceuticals/biontech-build-mrna-manufacturing-site-singapore-2021-05-10/.
65 Marimi Kishimoto, “Thai king-owned biotech starts production of AstraZeneca vaccine,” Nikkei Asia, June 4, 2021, https://asia.nikkei.com/Spotlight/Coronavirus/COVID-vaccines/Thai-king-owned-biotech-starts-production-of-AstraZeneca-vaccine.
66 Primus Dorimulu, Maria Fatima Bona, “Indonesia administers Sinovac vaccine manufactured by Bio Farma,” Jakarta Globe, February 19, 2021, https://jakartaglobe.id/news/indonesia-administers-sinovac-vaccine-manufactured-by-bio-farma; Tom Allard and Kate Lamb, “Indonesia in talks with WHO to become global vaccine hub: minister,” Reuters, September 16, 2021, https://www.reuters.com/world/asia-pacific/exclusive-indonesia-talks-with-who-become-global-vaccine-hub-minister-2021-09-16/.
67 “Taiwan says AstraZeneca COVID shot production talks fell through,” Reuters, June 10, 2021, https://www.reuters.com/business/healthcare-pharmaceuticals/taiwan-says-astrazeneca-covid-shot-production-talks-fell-through-2021-06-10/.
68 Makoto Kosaka et al. “Delayed COVID-19 vaccine roll-out in Japan.” The Lancet 397, no. 10292 (2021), https://doi.org/10.1016/S0140-6736(21)01220-4.
69 Stephen Croucher, Doug Ashwell and Jo Cullinane, “New Zealand has ramped up vaccination rates, but too many people remain concerned about vaccine safety,” The Conversation, September 20, 2021, https://theconversation.com/new-zealand-has-ramped-up-vaccination-rates-but-too-many-people-remain-concerned-about-vaccine-safety-167984.
70 The WHO’s interim lists of National Regulatory Authority evaluation results can be found at https://www.who.int/initiatives/who-listed-authority-reg-authorities; See “List of National Regulatory Authorities (NRAs) operating at maturity level 3 (ML3) and maturity level 4 (ML4) (as benchmarked against WHO Global Benchmarking Tool (GBT),” https://www.who.int/initiatives/who-listed-authority-reg-authorities/MLA4 and “List of vaccine producing countries with functional NRAs,” https://www.who.int/initiatives/who-listed-authority-reg-authorities/list-of-vaccine-prod-countries.
71 Stephanie Nolen, “Here’s why developing countries can make mRNA vaccines,” The New York Times, October 22, 2021, https://www.nytimes.com/interactive/2021/10/22/science/developing-country-covid-vaccines.html.
72 The detailed explanation on sources of COVID-19 dataset maintained by Our World in Data can be found at https://github.com/owid/covid-19-data/tree/master/public/data.
73 Hannah Ritchie, Edouard Mathieu, Lucas Rodés-Guirao, Cameron Appel, Charlie Giattino, Esteban Ortiz-Ospina, Joe Hasell, Bobbie Macdonald, Diana Beltekian and Max Roser, “Coronavirus Pandemic (COVID-19),” OurWorldInData.org, 2020, https://ourworldindata.org/coronavirus.